![]() > ๊ธฐ์ ์๊ฐ > ํ๊ฒฝPLANT์์ค > ํ์์ฒ๋ฆฌ์์ค
> ๊ธฐ์ ์๊ฐ > ํ๊ฒฝPLANT์์ค > ํ์์ฒ๋ฆฌ์์ค
- ๊ณ ํจ์จ ํ๊ธฐ์ฑ ์ํ
- ์ ์ด์ฐํ๋ฒ
- ํ์งยทํ์ธ๊ณต์
- ์ถ์ฐํ์์ฒ๋ฆฌ๊ณต์
- ์ค๊ธ์ ๋ฐ CNํ์์ฒ๋ฆฌ๊ณต์
ํ์ง,ํ์ธ์ฒ๋ฆฌ
ใ์ธ์ ์ฒญ์ ์ ํ์งํ์ธ์ฒ๋ฆฌ๊ณต์ ์ ์ข
๋์ ๊ธฐ๋ณธ ์ง์ฐํ ๋ฐ ํ์ง๋ฐ์์ ๋ถ๊ฐํ ANAMOX์ SURAMOX๋ฐ์์
๋ณํํ์ฌ ์ผ์ด๋๊ฒ ํ๋ฏ๋ก ์ ๋ ฅ๋น์ ํ์์์ ์ ์ฝํ์ฌ ์ด์ ๋น๋ฅผ ์ต์ํํ๋ ํ์ฐจ์ ๋์ ๊ณต์ ์
๋๋ค.
๊ณต์ ๋
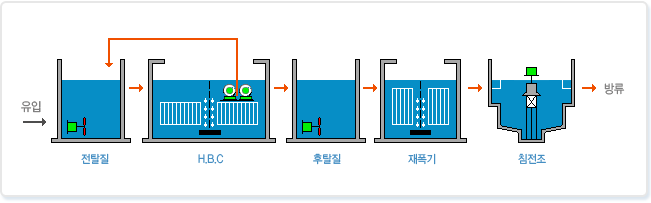
๋ฐ์๋งค์นด๋์ฆ
์๋ชจ๋์ํ
์ ๊ธฐ๋ฌผ์ง ๋ด ํจ์ ๋ ์ง์๋ ๋๋ถ๋ถ์ด ํ์ํ ์๋ฏธ๋
ธํ๋ก ์กด์ฌํ๋, ๋ฏธ์๋ฌผ์ด ์ ๊ธฐ ์ง์๋ฅผ ์๋ชจ๋์ํํ๋ ๊ณต์ .
CHONSโCO2+H2O+NH3 +์ธํฌ์ฆ์
์ง์ฐํ
์๋ชจ๋์ ๋๋ ์๋ชจ๋ ์ด์จ์ ์ ๊ธฐ๋ฌผ์ด ์๋ ์ํ์์ ํ์ฐ๊ฐ์ค, ํ์ฐ ๋ฐ ์คํ์ฐ์ ํใ
์์์ผ๋ก ์์ง์ฐ๊ท ๋ฐ ์ง์ฐ๊ท ์ ์ํด ์ง์ฐํํ๋ ๊ณต์ .
2NH4 +3O2 + HCO2- โ 2NO2 +2H2O + 4H+ +์ธํฌ
NO2- + O2 +HCO3- โ 2NO2 +2H2O + 4H+ +์ธํฌ
์ด๊ด : NH4+ + 2O2 โ NO2- + H2O+2H+
ํ์งํ
ํ์ง์ธ๊ท ์ ์์, ํ๊ธฐ์ฑ์ด๊ณ O2 ๊ฐ ์กด์ฌํ๋ฉด ์ด๋ฅผ ์ด์ฉํ์ฌ ํธํกํ๋, ๋ฌด์ฐ์ ์กฐ๊ฑด ํ์์ NO2, NO3 ๋ด์ O๋ฅผ ์ด์์์ฌ ํธํกํจ.
2NO3- + 10H+ +CH3OH โ N2 +4H2O + 2OH- +์ธํฌ
2NO2 +6H+ +CH3OH โ N2 +2H2O + 2OH- +์ธํฌ
๊ฒฐํฉ๋ ํ์ง๋ฐ์
-ANAMOX
NH4+ + 3/2NO3- 1/2N2 + 3/2NO2- + H+ + 3/2H2O
NH4+ + 3/5NO3- 4/5N2 + 2/5N2 + 9.5H2O
NH4+ + 6/5NO3- NO2- +3/5N2 +4/5H+ + 8/5H2O
NH4+ + NO2- N2 + 2H2O
-SURAMOX
NO3- + 5/3So +1/3H2O 1/2N2 +5/6(SO)ยฒ- + 2/3H+
NO2- + 1/2So 1/2N2 +1/2(SO)ยฒ-
NO3- +5/8HS- + 3/8H+ 1/2N2 +5/8(SO)ยฒ- + 1/2H2O
NO2- +3/8HS- + 5/8H+ 1/2N2 +5SO + 3H2O
NO3- +5HS- +6H+ 1/2N2 +3/8(SO)ยฒ- + 1/2H2O
NO2- +3HS- + 4H+ 1/2N2 +3SO + 2H2O
ํน์ง
HBC์กฐ๋ด ์ง์ฐํ๋ฏธ์๋ฌผ ๊ณ ๋๋ ๋ณด์ ๋ก ์ง์ฐํ ๋ฅ๋ ฅ์ด ํ์ํฉ๋๋ค.
๋ฉํ์ฌ ์ฌ์ฉ๋์ ์ต์ํํฉ๋๋ค.
์ ํ์ง์์์ BOD, COD์ ์ ๊ฑฐ๋ก HBC์กฐ ์ฒ๋ฆฌ๋ฅ๋ ฅ์ด ์ปค์ง๋๋ค.
์ ์ฉ๊ฐ๋ฅํ์
์ง์ํจ์ ํ์
์ ์ฉ๋ถ์ผ
์ํํ์, ์ ์ฝํ์, ๋ฐํจํ์, ์ฃผ์ ํ์, ์ ์ฝํ์, ์์ ํํํ์์ธ ์ค์, ํ์